Chemistry is an experimental science, therefore it is necessary to be able to work with units and measurements accurately.
Metric System
The metric system is based on prefixes that indicate a power of 10 with base units.
Metric Prefixes commonly used in chemistry |
Prefix |
Symbol |
Multiple |
Kilo |
k |
1000 |
Deci |
d |
0.1 |
Centi |
c |
0.01 |
Milli |
m |
0.001 |
Micro |
m |
0.000001 |
Nano |
n |
0.000000001 |
SI System
The International System of units gives a standard unit for each type of measurement.
SI Units commonly used in chemistry |
Measurement |
Unit |
Symbol |
Mass |
Kilogram |
kg |
Volume |
Liter |
L |
Temperature |
Kelvin |
K |
Length |
Meters |
m |
Time |
Seconds |
s |
Amount of substance |
Mole |
Mol |
Energy |
Joule |
J |
Charge |
Coulomb |
C |
There are also some important non-SI units as well.
Non-SI Units commonly used in chemistry |
Measurement |
Unit |
Symbol |
Length |
Anstrom |
Å |
Pressure |
Atmosphere |
Atm |
Kilopascal |
kPa |
|
Energy |
Calorie |
cal |
Temperature |
Celcius |
°C |
Taking measurements
Measurements must be taken accurately. Always write down one more decimal place than the instrument tells for certain—a “0” if it’s “one the line” and a “5” if it’s “between the lines.”
Lab Safety
There are several important safety rules that should be followed at all times:
- Wear splash-proof goggles
- No eating or drinking
- Do not touch, tast or directly smell chemicals
- Tie back all loose clothing, hair and jewelry
- Always read the procedure ahead of time and follow it closely
- Never return unused chemicals to the original container
- Dispose of all chemicals as instructured
- Always report all incidents (spills, breakage, mistakes in performing a procedure) to our instructor!
Common Lab Procedures
Some common lab techniques:
- Always point the end of the test tube away from people when heating
- Waft smells towards you to smell
- Add acid to water to prevent concentrated acid splashes
- Use a fume hood when working with toxic fumes
- Never mouth pipette
Tips for finding mass: Use a balance, don’t put hot things on the balance, don’t put chemicals directly on the balance, record exact mass. When finding volumes, use a graduated cylinder, read with level at eye level (you go to its level), record exact amount.
Some common techniques for separating mixtures: filtration (solid & liquid), chromatography (liquids based on attractions or size) and distillation (liquids based on boiling points). Some other common lab techniques are calorimetry—indirectly measuring energy changes, density—measure mass and volume of several samples, and titration—Find concentration of unknown solution.
Significant Figures
The significant figure rules are to allow people to read data or calculations and know with what precision the data was taken. The significant rules can be summarized in two rules: (1) If a decimal point is not present, count digits starting with the first the first non-zero number and ending with the last non-zero number; (2) If a decimal point is present anywhere in the number, start counting with the first non-zero number and continue until the end of the number. Rules on how to perform calculations with significant figures will be given in a future tutorial.
Fundamental Constants
Several numbers are used throughout chemistry and are important to be familiar with.
Fundamental constants commonly used in chemistry |
Name |
Symbol |
Constant |
Avogadro’s # |
NA |
6.02 X 1023 mol-1 |
Speed of light |
c |
3.0 X 108 m/s |
Gas constant |
R |
8.31 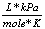 |
|
0.0821 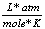 |
Planck’s constant |
h |
6.63 ´ 10-34 J·s |
Charge of electron |
e |
1.6 ´ 10-19 C |
Atomic mass unit |
m |
1.66 ´ 10-24 g |
Std Temp & Pressure |
STP |
273.15 K & 1 atm |