Physical and chemical changes occur with changes in heat.
Energy, heat and temperature
Several definitions are useful in understanding thermodynamics:
- Energy: The ability to do work or supply heat.
- System: Particles under-going change.
- Surroundings: Everything surrounding the system.
- Temperature: proportional to the average kinetic energy of the particles.
- Heat (q): Flow of energy from a hotter object to a cooler object.
- Enthalpy (H): Takes into account internal energy, pressure and volume. In an open-air environment, it’s the same as heat.
Physical changes
The specific heat capacity is the amount of energy that can be absorbed before temperature begins to change. 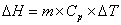 where m = mass; Cp = specific heat capacity and DT = T2 – T1. During changes in state, the heat of fusion and heat of vaporization are used. Melting:
where m = mass; Cp = specific heat capacity and DT = T2 – T1. During changes in state, the heat of fusion and heat of vaporization are used. Melting: 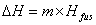 Hfus = enthalpy of fusion. Boiling:
Hfus = enthalpy of fusion. Boiling: 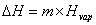 Hvap = enthalpy of vaporization. Heating curves show the combination of the processes—changing temperatures and changing states.
Hvap = enthalpy of vaporization. Heating curves show the combination of the processes—changing temperatures and changing states.
Chemical changes
The heat of formation is the energy change when a compound is formed from its elements. Hess’s law says that since energy is a state function, the path doesn’t matter—only where you began and ended, then the heat of a reaction can be found by adding up stepwise reactions that add up to the overall chemical reaction. This allows the heat of reaction to be found from formation values: 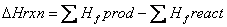
Calorimetry
When the system loses energy, the surroundings gain it and vice versa. Therefore, the energy change in the surrounding can be measured and used to determine information about the system. Calorimetry can be used for physical or chemical processes.
Enthalpy, Entropy and Free energy
Entropy is disorder or randomness. All spontaneous processes result in a net increase in entropy for the universe. The spontaneity of a process (shown with a negative free energy value) is found by relating enthalpy, entropy and temperature to find free energy. 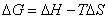
| Enthalpy |
Entropy |
Spontaneous at |
+ |
+ |
High temps |
+ |
- |
Never |
- |
- |
Low temps |
- |
+ |
All temps |